FDA approves first postpartum depression pill

The Food and Drug Administration approved the drug, called Zurzuvae, for adults experiencing severe depression related to childbirth or pregnancy. It's taken once a day for 14 days.

FDA approves first oral treatment for postpartum depression - Pharmaceutical Technology

Fda Approves First Postpartum Depression Pill Stock Photo
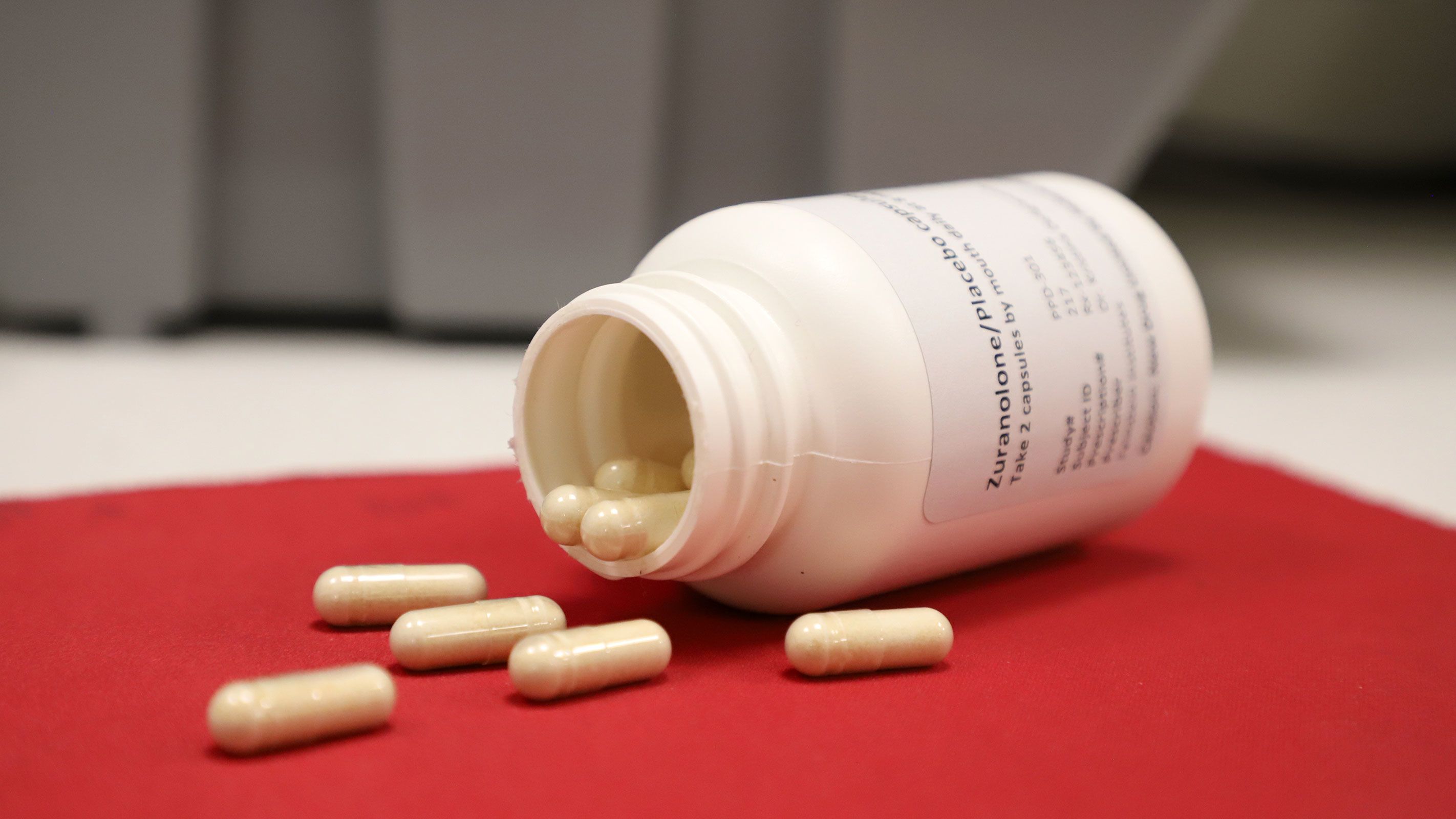
FDA approves first postpartum depression pill in the US

FDA Approves First Pill for Postpartum Depression - The New York Times
FDA approves pill to treat postpartum depression, Lifestyles

Zuranolone FDA approval adds treatment option for women with

Zuranolone: First FDA-Approved Pill for Postpartum Depression

FDA Approves ZURZUVAE™ (zuranolone), the First and Only Oral

FDA approves first postpartum depression pill - The Standard Health

FDA For The Baby Blues: First Pill for Postpartum Depression - Fortunes Crown

FDA approves first postpartum depression pill

Insight: FDA approves first postpartum depression pill in U.S. – KXAN Austin

FDA approves first postpartum depression pill in the US







