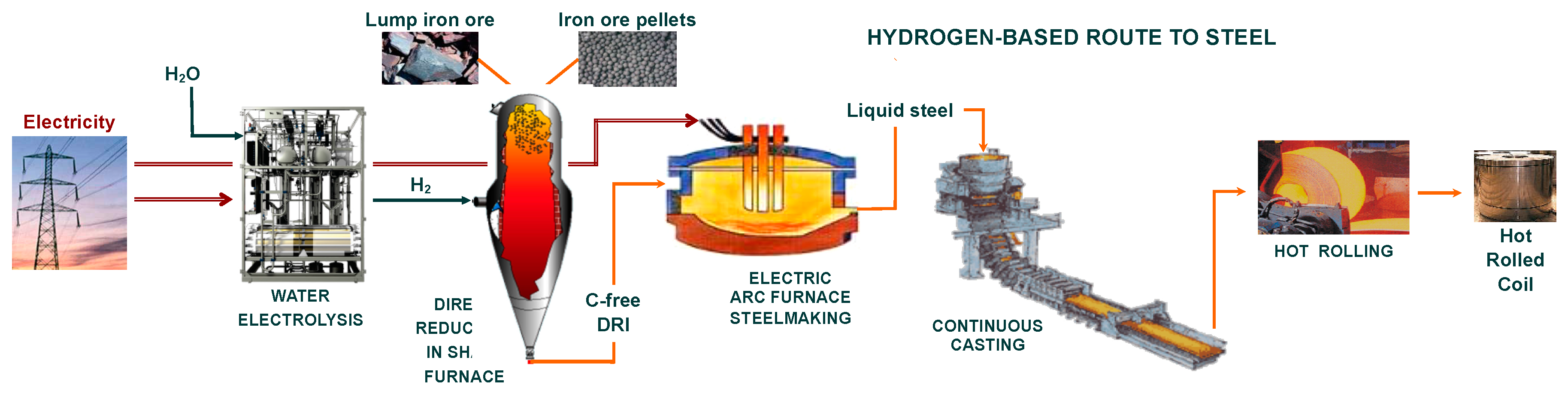
A new route for making steel from iron ore based on the use of hydrogen to reduce iron oxides is presented, detailed and analyzed. The main advantage of this steelmaking route is the dramatic reduction (90% off) in CO2 emissions compared to those of the current standard blast-furnace route. The first process of the route is the production of hydrogen by water electrolysis using CO2-lean electricity. The challenge is to achieve massive production of H2 in acceptable economic conditions. The second process is the direct reduction of iron ore in a shaft furnace operated with hydrogen only. The third process is the melting of the carbon-free direct reduced iron in an electric arc furnace to produce steel. From mathematical modeling of the direct reduction furnace, we show that complete metallization can be achieved in a reactor smaller than the current shaft furnaces that use syngas made from natural gas. The reduction processes at the scale of the ore pellets are described and modeled using a specific structural kinetic pellet model. Finally, the differences between the reduction by hydrogen and by carbon monoxide are discussed, from the grain scale to the reactor scale. Regarding the kinetics, reduction with hydrogen is definitely faster. Several research and development and innovation projects have very recently been launched that should confirm the viability and performance of this breakthrough and environmentally friendly ironmaking process.

Metals (2)

Mistborn Allomantic Metals as coins by Mirimeh, Download free STL model

Metals, Free Full-Text
Metals, Free Full-Text
Chancey Metals

The Vault Metals Storage Program - Money Metals Exchange

SA Metal recycling
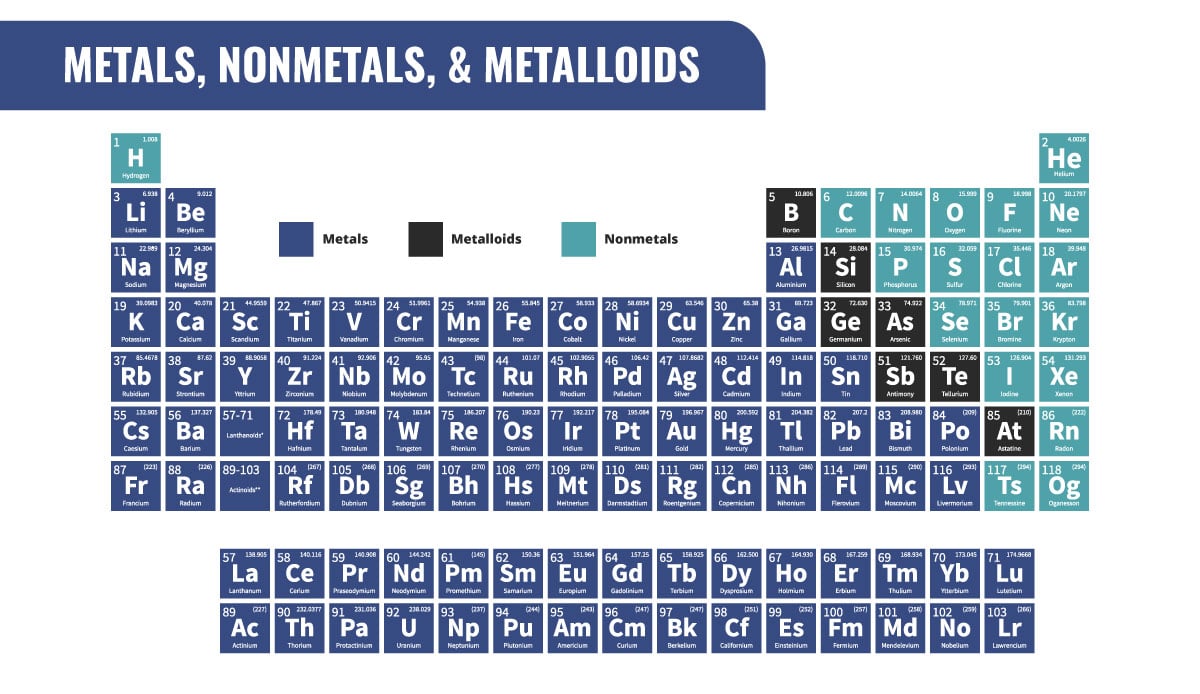
What's the Difference Between Metals, Nonmetals, and Metalloids?
Metals, Free Full-Text







:max_bytes(150000):strip_icc()/cdn.cliqueinc.com__cache__posts__277309__short-haircuts-for-women-over-40-277309-1549952142152-image.700x0c-e4004f1066dd4f1d90a96745ba548a52.jpg)