Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal Spacer, Proven Safe and Effective at Minimizing the Harmful Long-Term Side Effects of Prostate Radiation Therapy - Palette Life Sciences

Groundbreaking technology introduces increased control to achieve optimal coverage proven to significantly reduce the risk of toxicity to the rectum SANTA BARBARA, CALIF. / STOCKHOLM, SWEDEN – June 9, 2022— Palette Life Sciences, a fully-integrated global life sciences company dedicated to improving patient outcomes, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of […]
Barrigel Proven Safe & Effective at Minimizing Side Effects

Israeli firm gets FDA approval for rectum shield that blocks
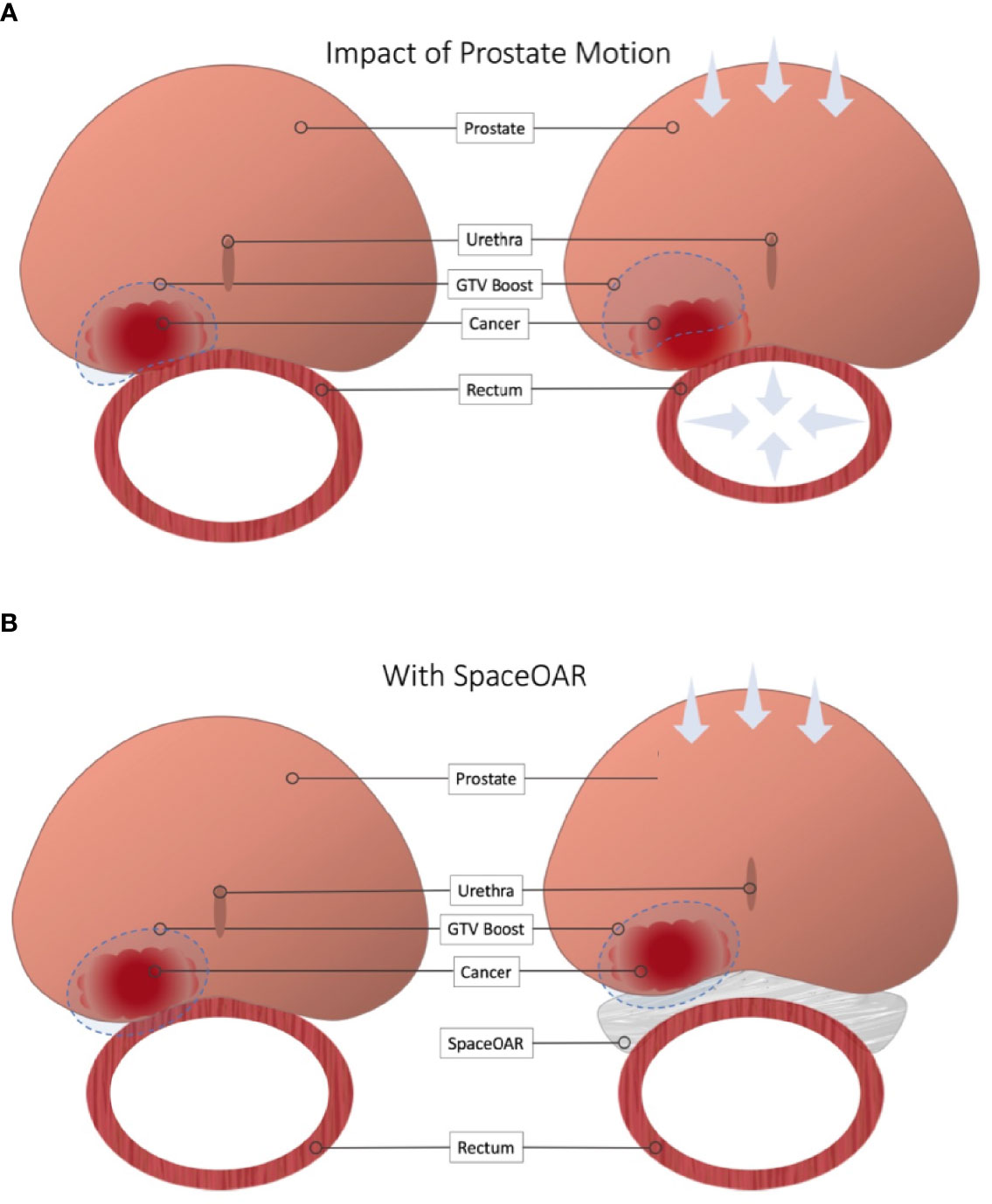
Frontiers Rationale for Utilization of Hydrogel Rectal Spacers

James Leech on LinkedIn: Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel® Rectal…

Barrigel on LinkedIn: Palette Life Sciences Announces FDA 510(k
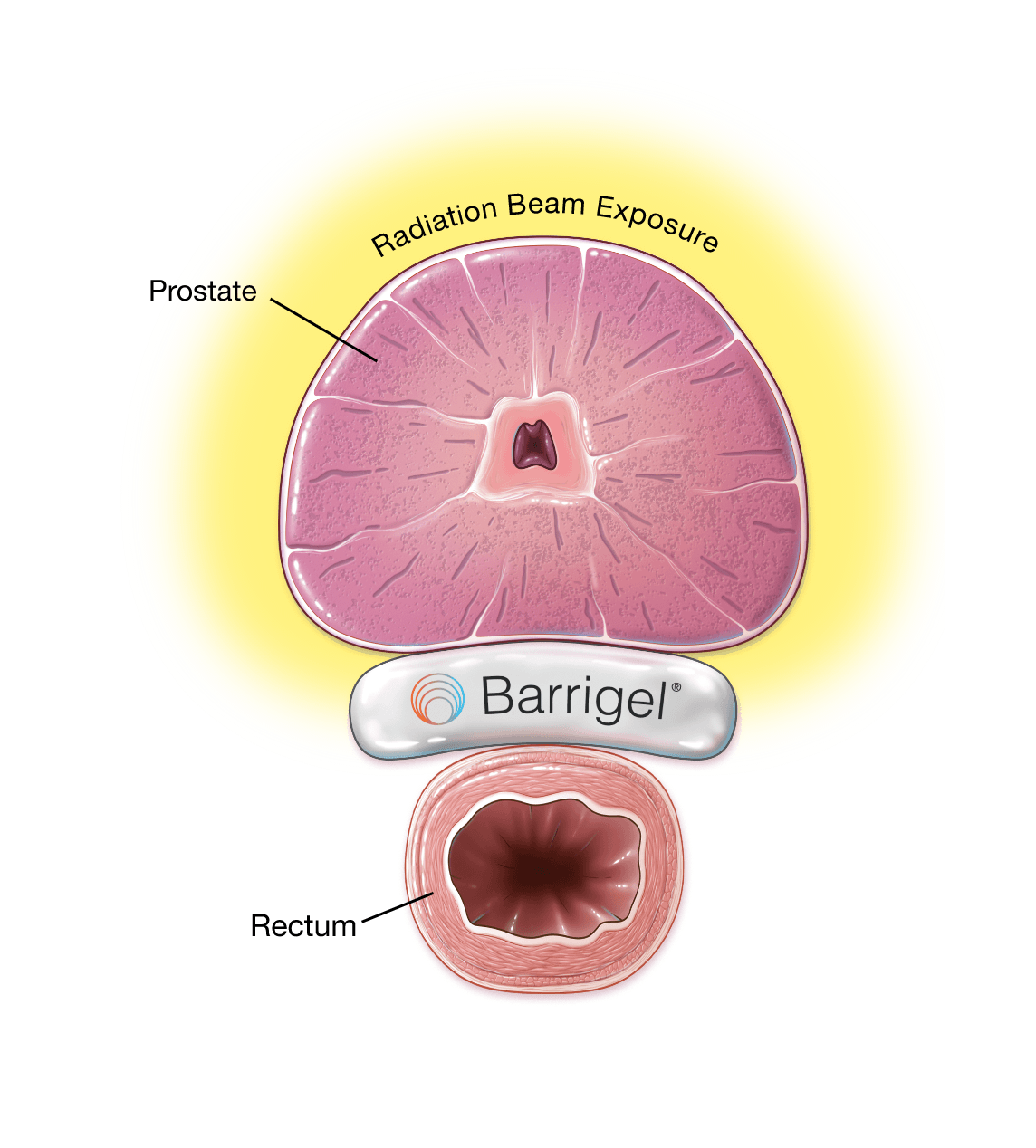
Barrigel Spacer Minimize Prostate Radiation Side Effects
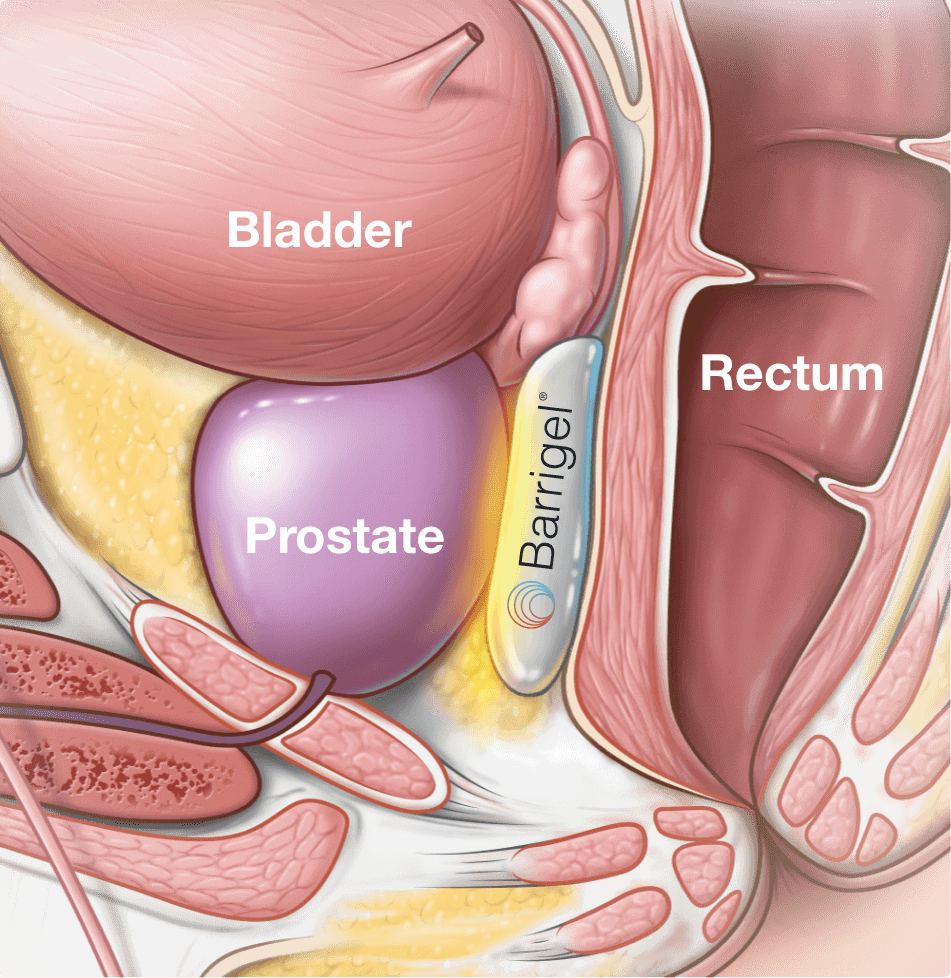
About Your Barrigel Procedure for Prostate Cancer Radiation

FDA clears BioProtect Balloon for rectal protection during
Barrigel Proven Safe & Effective at Minimizing Side Effects







