20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as
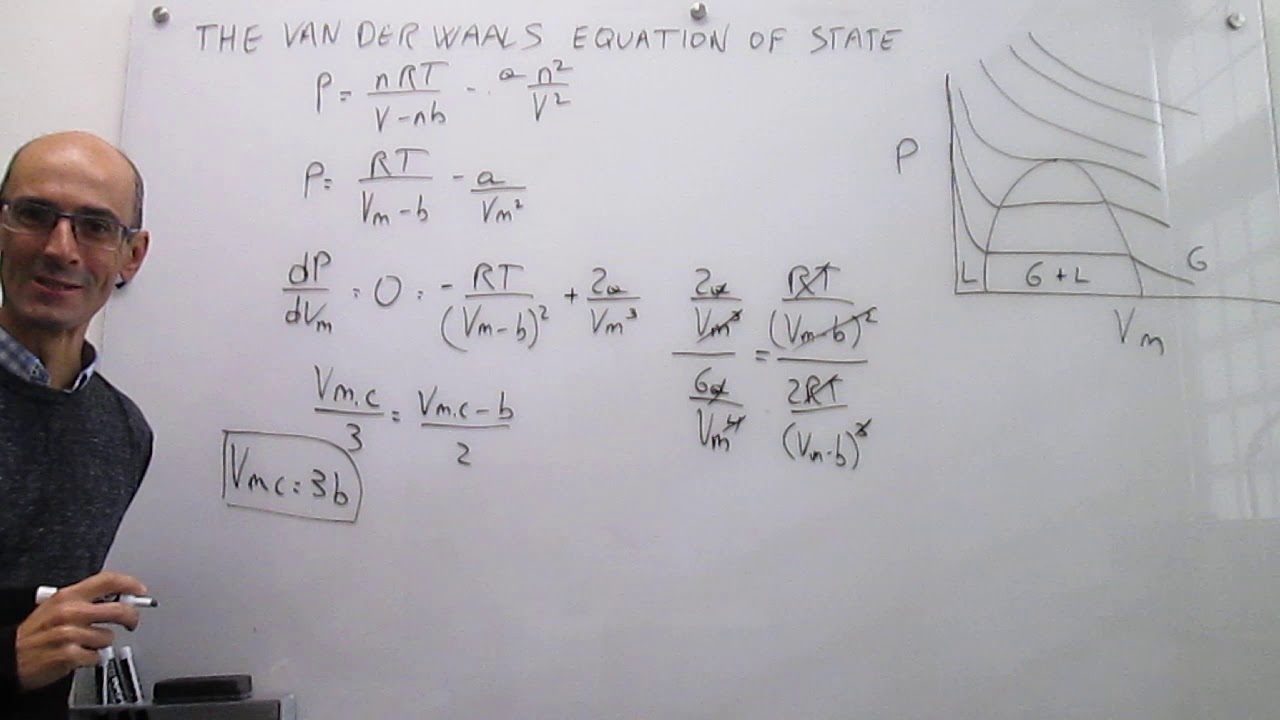
The van der Waals equation of state at the critical point

20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as

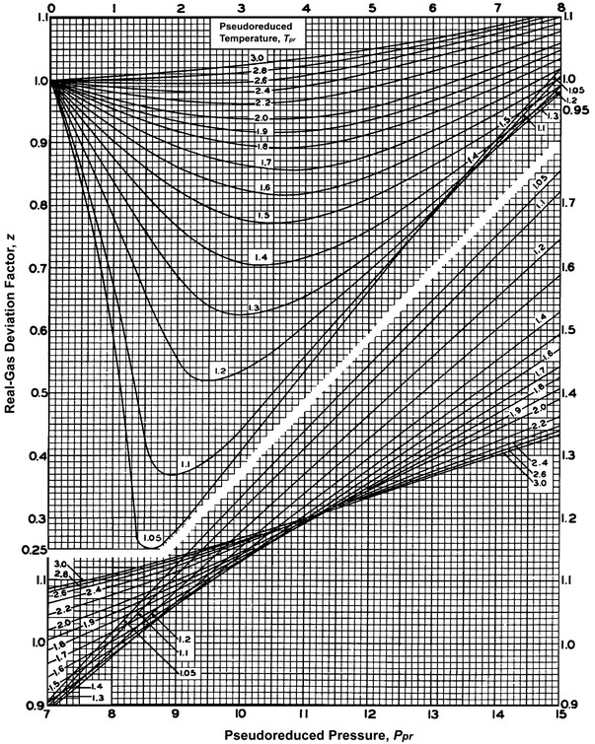
Compressibility Chart - an overview

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
Bengali] The compresibility factor (Z) of one mole of a van der waals
The compressibility factor for one mol of a vanderwalls gas at 0 degree c and 100atm pressure is .5 then what will be the volume of 2 mols of this gas

Objectives_template

Which of these are correct? A) Z, compressibility factor, low pressure can be written as z = B) Z, low pressure can be written as z = 1 + P C) Z

Compressibility Factor Calculator - File Exchange - MATLAB Central
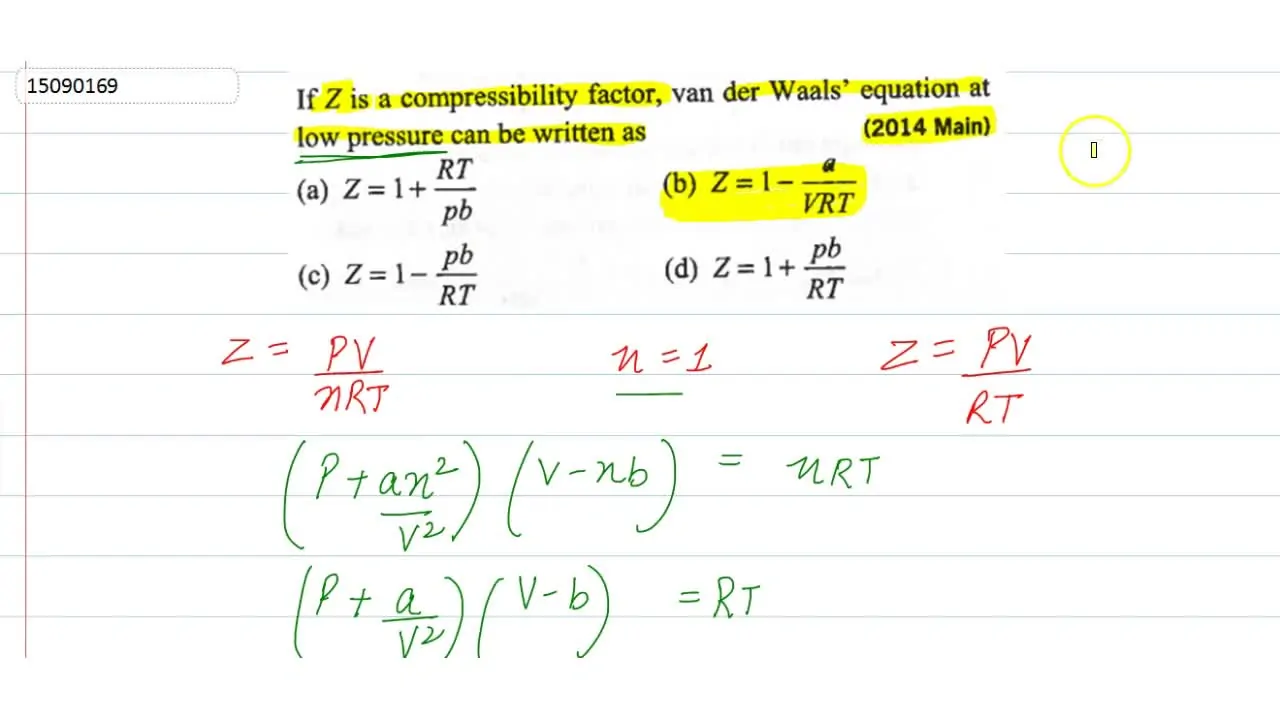
If Z is a compressibility factor, van der Waals' equation at low press

Punjabi] What is the compressibility factor (Z) for 0.02 mole of a va

At low pressure the van der Waals' equation is reduced to [P +(a)/(V^(
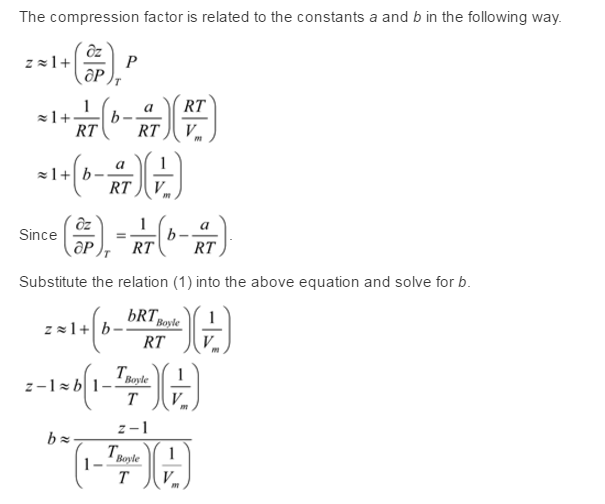
Solved) - For values of z near 1, it is a good approximation to write z(P) = - (1 Answer)